Explain Why All Three Net-ionic Equations Are Different
Explain why any ions are cancelled as spectators. Write the balanced molecular equation for the reaction including the state of each substance.

Net Ionic Equation Worksheet And Answers Youtube
The key difference between molecular equation and ionic equation is that the molecular equation shows the reactants and products in molecular form while the ionic equation shows the ionic species involved in the reaction.

. APPLICATION OF PRINCIPLES Classify each of the following reactions as 1 combination 2 decomposition 3 displacement or 4 metathesis. A net ionic equation only shows the chemical species elements compounds ions which are directly involved in the chemical reaction. 025 aqis combined with a 500 mL sample of 025 MHCOOHaq.
For example we could have sodium chloride. Work as a group to write a definition for a net ionic equation. The result of making all these replacements is the complete ionic equation.
Neutralization Reactions and Net Ionic Equations for Neutralization Reactions. Reacts according to the equation above. Up to 24 cash back 7.
Explain why the net ionic equation for the neutralization reaction between HClaq and KOHaq is the same as the net ionic equation for the neutralization reaction between HNO3aq and RbOHaq. Explain why it is valid to remove this species from the equation. Be familiar with the experimental steps and what was observed in the experiment in which crystals of silver nitrate and potassium chloride were pushed into a puddle Section D.
IWrite the balanced net ionic equation for the reaction that occurs when H. Explain why it is valid to remove this species from the equation. Write the balanced net ionic equation for the reaction of Cl and AgNO3.
Is combined with HCOOH. Compare the net ionic equation in Model 2 to the other two equations. SPCCCS Q CL poviå- JrÁL YCQCfiovx STOP.
In your own words please describe the difference between a complete ionic equation and a net ionic equation. There are different ways to write equations for chemical reactions. Explain in your own words.
The chemical equation shows what the reactants are and what are the products. In the third line the full ionic equation is repeated but cancellation of species appearing the same on both sides is indicated which leads to the final equation the net ionic equation. Explain why the net ionic equation for the neutralization reaction between HClaq and KOHaq is different from the net ionic equation for the neutralization reaction between HClaq and AgOH.
Aq K b 13 10. What chemical species is missing in the net ionic equation. Write the molecular and net ionic equations for the reaction of Na2S with HCI which takes place in aqueous solution.
Explain why the net ionic equation for the neutralization reaction. Write the complete and net ionic equations for the neutralization reaction between HClaq and KOHaq using the hydronium ion in place of H. What chemical species is missing in the net ionic equation.
Some of the most common are unbalanced equations which indicate the species involved. Chemistry QA Library Find the net ionic equation for 2Als 3CuCl22H2Oaq - 3Cus 2AlCl3aq 6H2Ol. Balanced chemical equations which indicate number and type of species.
The other ions that do not participate in a precipitate are known as the spectator ions so we could have different spectator ions in the reaction yet giving us the same net ionic equation. There are three steps in writing net ionic equation in the procedure. Write net ionic equations to explain the formation of a a white precipitate when solutions of calcium sulfate and sodium carbonate are mixed.
In this experiment you will be combining different aqueous solutions of ionic compounds in very small amounts 4-5 drops and then observing them for signs of a any reaction. And net ionic equations which only deal. Molecular equations which express compounds as molecules instead of component ions.
A 500 mL sample of M H. In cases where a reaction occurs an. B two different precipitates formed when solutions of ironIII sulfate and barium hydroxide are mixed.
6 d In aqueous solution the compound H. Explain why any ions are cancelled as spectators. Experiments 5 9 and 11 are reactions of an acid with a carbonate salt to yield CO2g and H2Ol yet all have different net ionic equations.
Sid S - did no chanqt 8. For the neutralization reaction between any monoprotic strong acid and strong. So when we show a Net Ionic equation were just showing the two ions that go together to make the precipitate.
Work as a group to write a definition for a net ionic equation. A net ionic equation is a reaction equation in which all spectator ions are removed leaving only the ions and molecules directly involved in the reaction. We can find the net ionic equation for a given reaction using the following steps.
A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions. Compare the net ionic equation in Model 2 to the other two equations. Does water completely ionize in solution.
Complete ionic equation and net ionic equation are two ways of representing a chemical reaction. The key difference between Complete ionic and net ionic equation is that complete ionic equation gives all the ionic species participated in the chemical reaction whereas net ionic reaction. Explain in your own words how this second reaction affects the equilibrium of the iron thiocyanate reaction and what this indicates about the stability of the ironIII fluoride complex compared to the ironIII thiocyanate complex.
Place the appropriate number in the blank at the left of the equation. Chemical reactions are interactions between chemical compounds to form new compounds or. Spectator ions are cancelled out in a net ionic equation in contrast to a complete ionic equation which includes spectator ions that remain unchanged and appear on both sides of the equation.
The aqueous sodium chloride that is produced in the reaction is called a salt. Explain why all three net-ionic equations in Steps 1 2 and 3 are different. October 13 2019 Posted by Madhu.
A neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water.

How To Write Net Ionic Equations In Chemistry A Simple Method Youtube
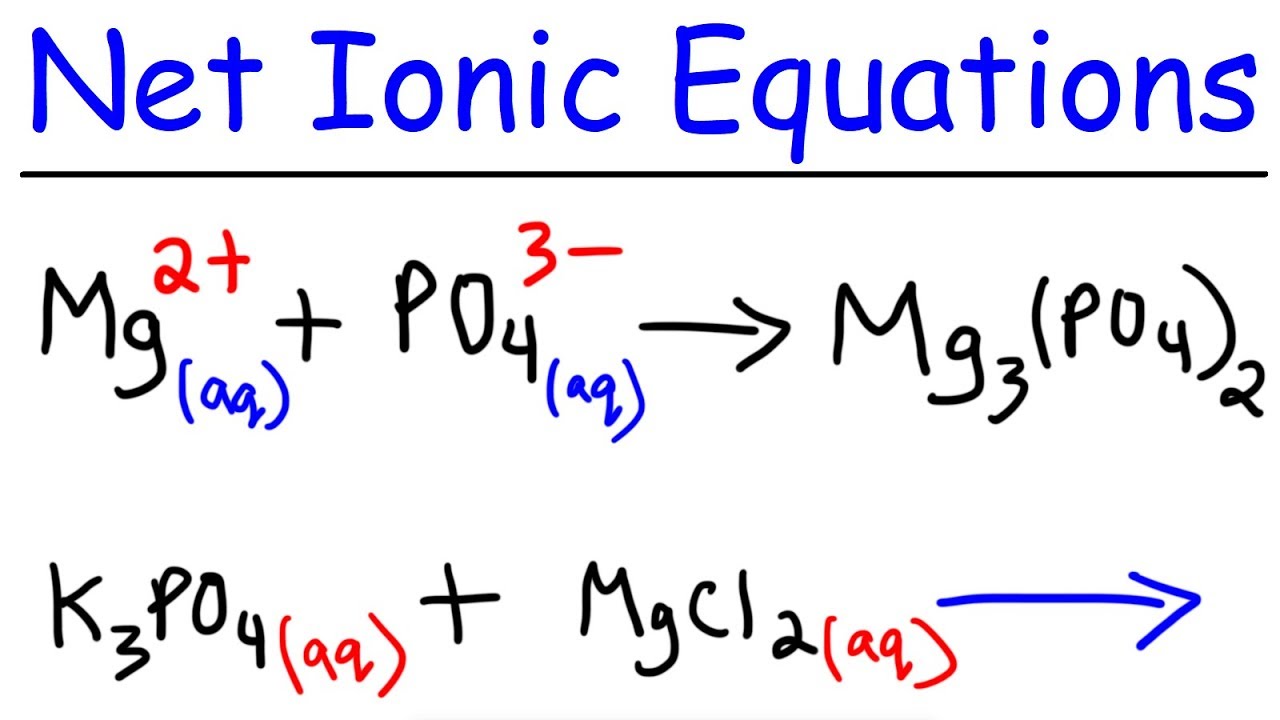
Precipitation Reactions Net Ionic Equations Chemistry Youtube

What Information Does The Complete Ionic Equation Give Lisbdnet Com
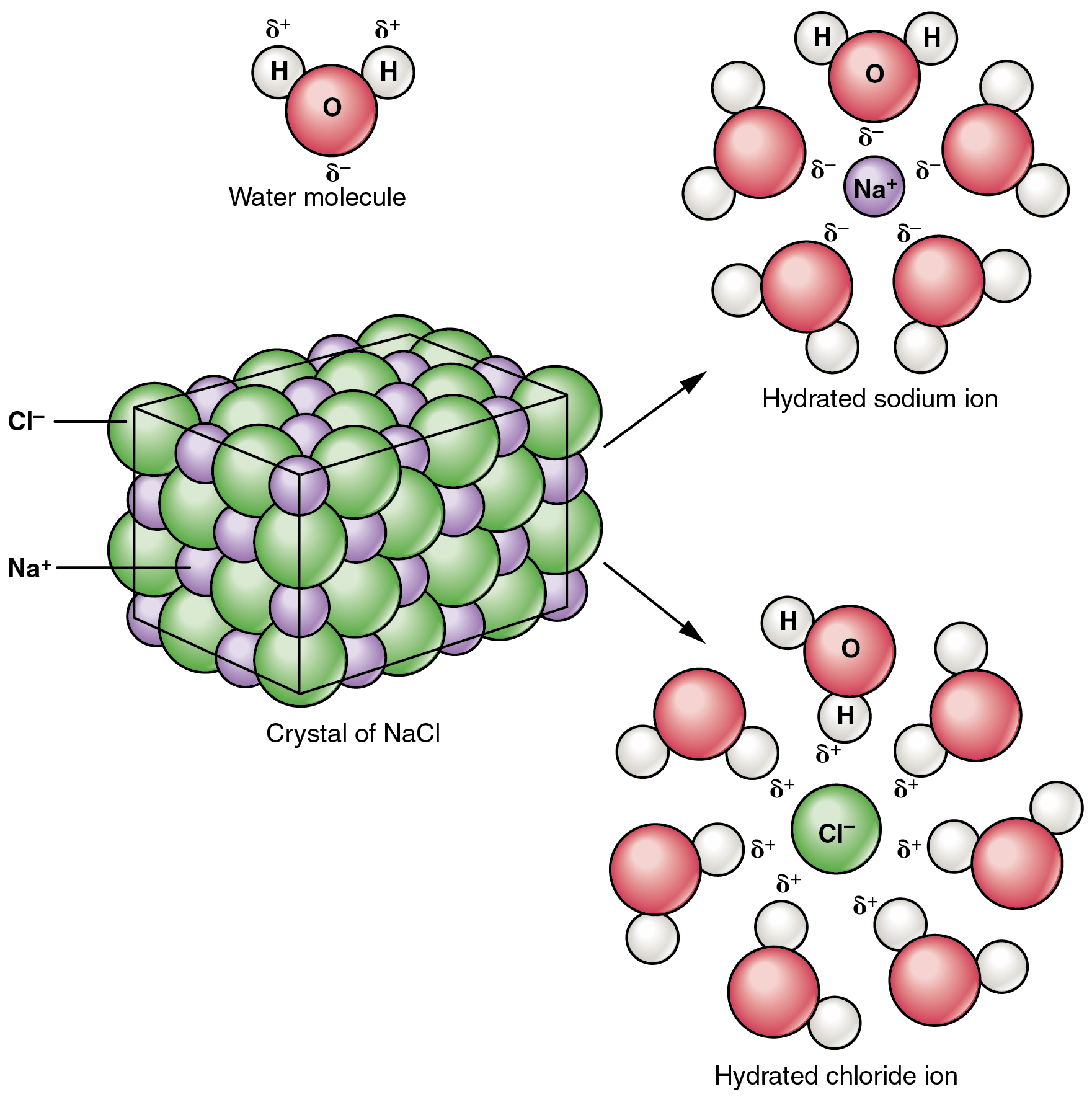
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy

Molecular Complete Ionic And Net Ionic Equations Video Khan Academy

4 3 Ionic Equations A Closer Look Chemistry Libretexts

Net Ionic Equation And Complete Ionic Equation
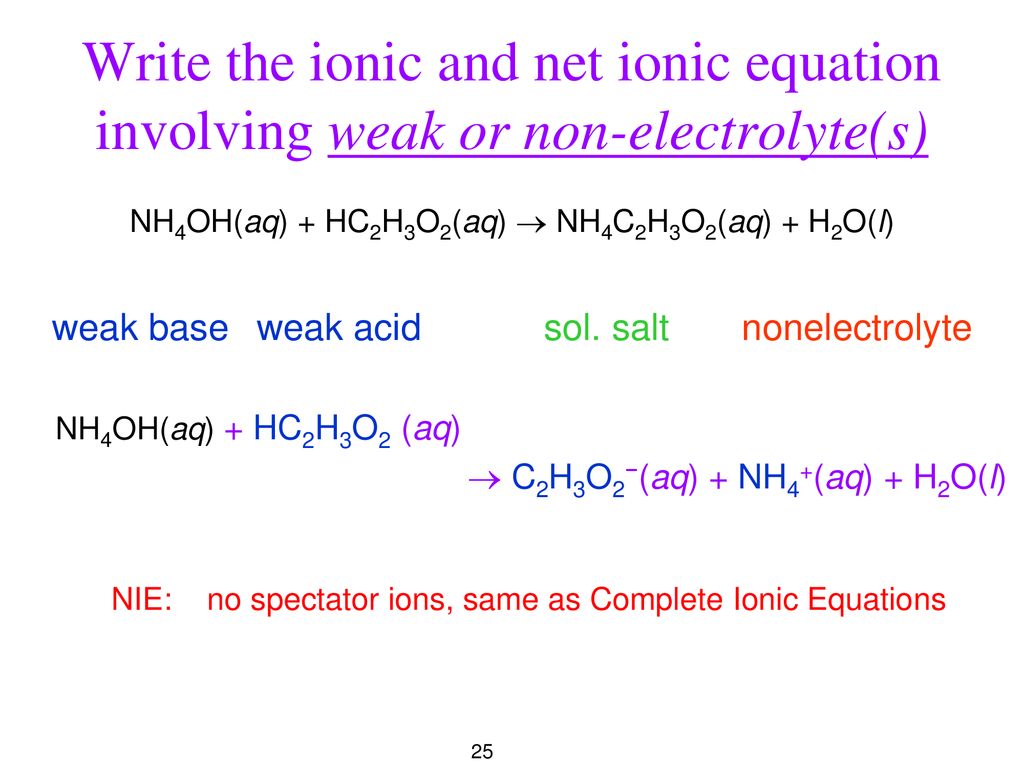
Complete Ionic Equation Net Ionic Equations Ppt Download

Rewriting A Complete Ionic Equation As A Net Ionic Equation Chemistry Study Com

Complete Ionic Equation Net Ionic Equations Ppt Download
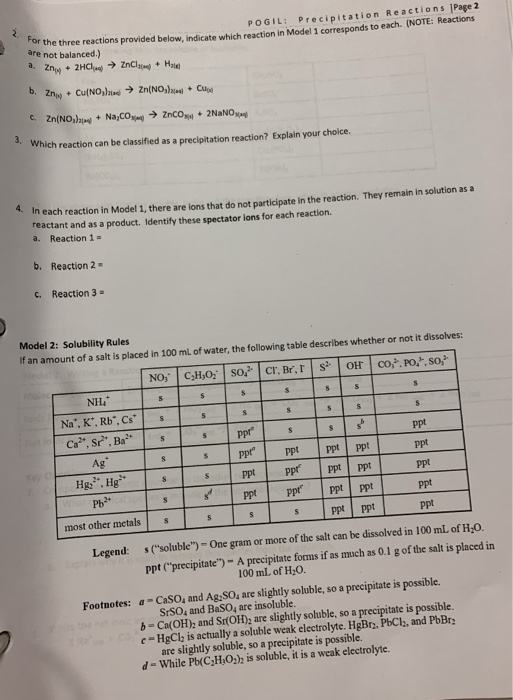
Solved Model 1 Three Reactions Types Of Reactions Ess Chegg Com
Net Ionic Equations Lab Ap Chemistry Shelly Oh

Rewriting A Molecular Equation As A Net Ionic Equation Chemistry Study Com

Difference Between Molecular Equation And Ionic Equation Compare The Difference Between Similar Terms

How To Write Net Ionic Equations Chemtalk
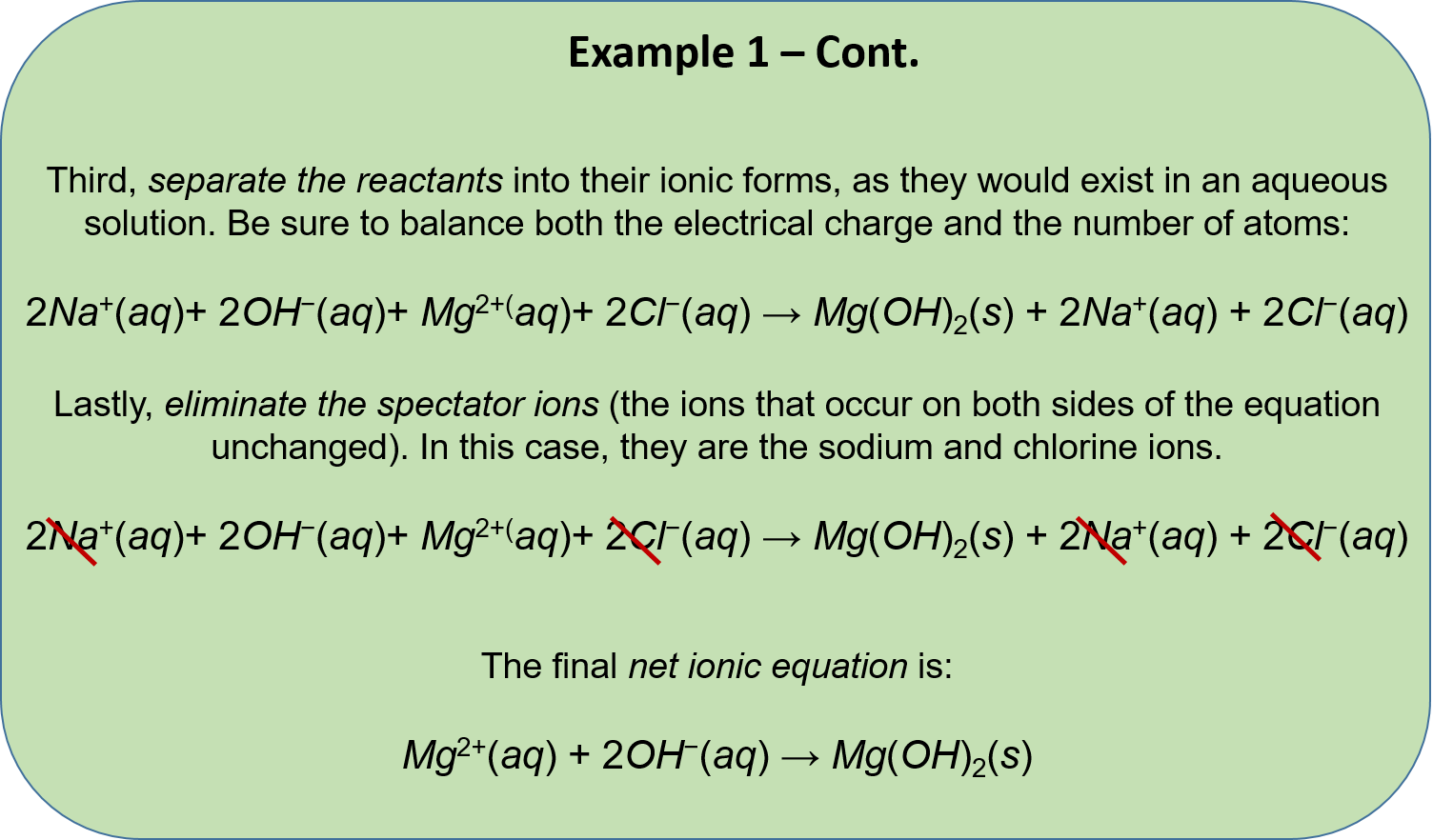
Ch104 Chapter 5 Chemical Reactions Chemistry
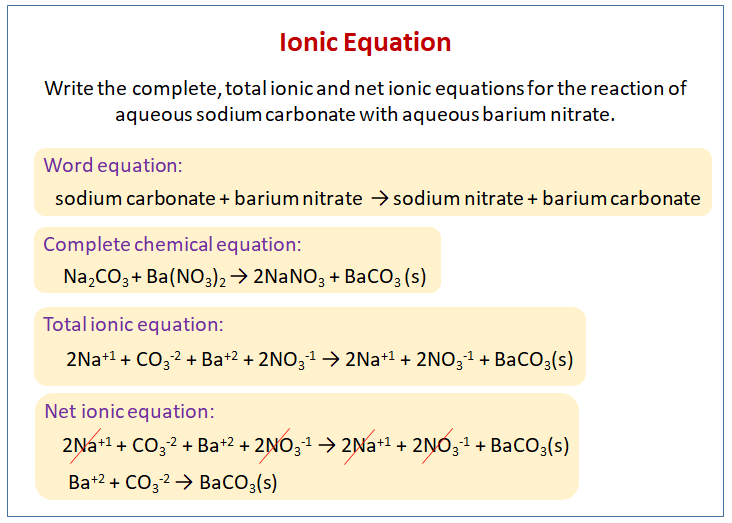
Writing Ionic Equation Video Lessons Examples And Solutions
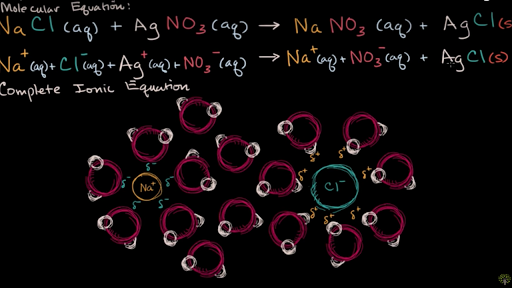
Molecular Complete Ionic And Net Ionic Equations Video Khan Academy

Solved Model 1 Three Reactions Types Of Reactions Ess Chegg Com
Comments
Post a Comment